About Constipation
About Constipation
Constipation is a widespread condition that impacts quality of life1
Constipation is a widespread condition that impacts quality of life1
41 million adults in the United States suffer from the discomfort of constipation each year2
Constipation is a common problem in patients with diabetes mellitus (DM)
While the exact mechanism of diabetic bowel dysfunction is not known, it’s suggested that neuropathy caused by hyperglycemia affects colon motility.8
NEUROPATHY8
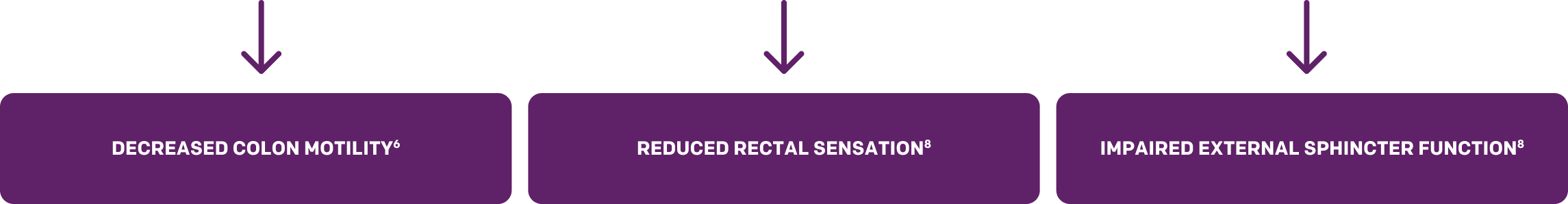
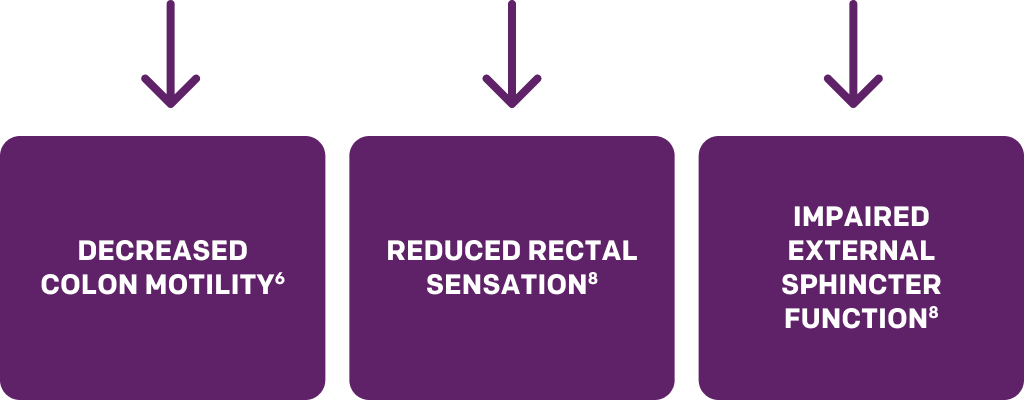
Hyperglycemia-induced diabetic neuropathy causes autonomic neuronal damage, nerve flow reduction, and vascular endothelium damage. Because autonomic neurons and smooth muscle are believed to regulate GI motility, diabetes increases the risk of constipation or hard stool as a result of decreased motility, bowel transmit time, and atony of the colon. Diabetic neuropathy also leads to reduced rectal sensation and impaired external sphincter function, which result in symptoms such as fecal urgency and feeling of incomplete evacuation.
NEUROPATHY8
Hyperglycemia-induced diabetic neuropathy causes autonomic neuronal damage, nerve flow reduction, and vascular endothelium damage. Because autonomic neurons and smooth muscle are believed to regulate GI motility, diabetes increases the risk of constipation or hard stool as a result of decreased motility, bowel transmit time, and atony of the colon. Diabetic neuropathy also leads to reduced rectal sensation and impaired external sphincter function, which result in symptoms such as fecal urgency and feeling of incomplete evacuation.
In a large, hospital-based cross-sectional study including 4738 patients of whom 603 were diabetic, the authors concluded that diabetes is associated with an increased risk of8:
|
| |
|
|
Additionally, they found that the risk of these symptoms is affected by8:
|
|
|
|
|
These symptoms remained associated even after the exclusion of organic gastrointestinal diseases.8
In a large, hospital-based cross-sectional study including 4738 patients of whom 603 were diabetic, the authors concluded that diabetes is associated with an increased risk of8:
- Constipation
- Fecal urgency
- Hard stools
- Incomplete evacuation
Additionally, they found that the risk of these symptoms is affected by8:
- High HbA1c levels
- Long duration of diabetes
- Low BMI
- High serum creatinine levels
- Use of insulin
These symptoms remained associated even after the exclusion of organic gastrointestinal diseases.8
Female patients are more likely than males to experience constipation9
Female patients are more likely than males to experience constipation9
Hormones, diet, supplemental iron, pregnancy, and menopause are all factors that may lead to constipation in women.10-14
Pregnancy is a common cause of constipation15
Pregnancy is a common cause of constipation15
Note that pregnant women are advised to talk to their doctors if they experience constipation during pregnancy to determine the best specific course of action for them.
Why is menopause often accompanied by constipation?
Physical changes associated with menopause affect all systems of women’s bodies, including the GI tract, often resulting in constipation.16
The connection between constipation and lower urinary tract symptoms21
An article in the Journal of Women’s Health reported that constipation was prospectively associated with increased risks of urgency and hesitancy in parous middle-aged women. The study suggests that untreated constipation could lead to the development of lower urinary tract symptoms (LUTS) in this patient population.21
An article in the Journal of Women’s Health reported that constipation was prospectively associated with increased risks of urgency and hesitancy in parous middle-aged women. The study suggests that untreated constipation could lead to the development of lower urinary tract symptoms (LUTS) in this patient population.21
If additional research supports a causal relationship, it implies that women should seek treatment for constipation to reduce their consequent risk of developing LUTS.21
References: 1. Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599-608. doi:10.1111/j.1365-2036.2006.03238.x 2. NIH. National Institute of Diabetes and Digestive and Kidney Diseases. Definitions & facts for constipation. Accessed May 7, 2024. https://www.niddk.nih.gov/health-information/digestive-diseases/constipation/definition-facts 3. Mayo Clinic. Constipation. Accessed May 7, 2024. https://www.mayoclinic.org/diseases-conditions/constipation/symptoms-causes/syc-20354253 4. Piper MS, Saad RJ. Diabetes mellitus and the colon. Curr Treat Options Gastroenterol. 2017;15(4):460-474. doi:10.1007/s11938-017-0151-1 5. Maisey A. A practical approach to gastrointestinal complications of diabetes. Diabetes Ther. 2016;7:379-386. doi:10.1007/s13300-016-0182-y 6. Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001;68(11):928-930, 932, 934-944. doi:10.3949/ccjm.68.11.928 7. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26(5):1553-1579. doi:10.2337/diacare.26.5.1553 8. Ihana-Sugiyama N, Nagata N, Yamamoto-Honda R, et al. Constipation, hard stools, fecal urgency, and incomplete evacuation, but not diarrhea is associated with diabetes and its related factors. World J Gastroenterol. 2016;22(11):3252-3260. doi:10.3748/wjg.v22.i11.3252 9. McCrea GL, Miaskowski C, Stotts NA, Macera L, Varma MG. A review of the literature on gender and age differences in the prevalence and characteristics of constipation in North America. J Pain Symptom Manage. 2009;37(4):737-745. doi:10.1016/ j.jpainsymman.2008.04.016 10. Vlitos A, Davies GJ. Bowel function, food intake and the menstrual cycle. Nutr Res Rev. 1996;9(1):111-134. doi:10.1079/NRR19960008 11. NIH. Office of Dietary Supplements. Iron. Fact sheet for health professionals. Accessed May 7, 2024. https://ods.od.nih.gov/factsheets/iron-HealthProfessional/ 12. Bradley CS, Kennedy CM, Turcea AM, Rao SSC, Nygaard IE. Constipation in pregnancy: prevalence, symptoms, and risk factors. Obstet Gynecol. 2007;110(6):1351-1357. doi:10.1097/01.AOG.0000295723.94624.b1 13. Oliveira SC, Pinto-Neto AM, Conde DM, et al. Constipation in postmenopausal women. Article in Portuguese. Rev Assoc Med Bras. 2005;51(6):334-341. 14. Rollet M, Bohn T, Vahid F. Association between dietary factors and constipation in adults living in Luxembourg and taking part in the ORISCAV-LUX 2 survey. Nutrients. 2021;14(1):122. doi:10.3390/nu14010122 15. Cleveland Clinic. Pregnancy constipation. Accessed April 28, 2024. https://my.clevelandclinic.org/health/diseases/21895-pregnancy-constipation 16. Rostami-Moez M, Masoumi SZ, Otogara M, Farahani F, Alimohammadi S, Oshvandi K. Examining the health-related needs of females during menopause: a systematic review study. J Menopausal Med. 2023;29(1):1-20. doi:10.6118/jmm.22033 17. Tack J, Müller-Lissner S, Stanghellini V, et al. Diagnosis and treatment of chronic constipation—a European perspective. Neurogastroenterol Motil. 2011;23(8):697–710. doi:10.1111/j.1365-2982.2011.01709.x 18. Magliano M. Menopausal arthralgia: fact or fiction. Maturitas. 2010;67(1):29-33. doi:10.1016/j.maturitas.2010.04.009 19. Kozinoga M, Majchrzycki M, Piotrowska S. Low back pain in women before and after menopause. Prz Menopauzalny. 2015;14(3):203-207. doi:10.5114/pm.2015.54347 20. Horn JR, Mantione MM, Johanson JF. OTC polyethylene glycol 3350 and pharmacists’ role in managing constipation. J Am Pharm Assoc. 2012;52(3):372-380. doi:10.1331/JAPhA.2012.10161 21. Alhababi N, Magnus MC, Drake MJ, Fraser A, Joinson C. The association between constipation and lower urinary tract symptoms in parous middle-aged women: a prospective cohort study. J Womens Health. 2021;30(8):1171-1181. doi:10.1089/jwh.2020.8624
Diagnostic tools: Distinguishing occasional vs chronic constipation
The criteria below may help when diagnosing patients1,2
While there’s no formal definition for occasional constipation, it can include1:
- Infrequent bowel movements
- Difficulty in passing stool
- Straining
- The feeling of incomplete evacuation
Chronic constipation is defined by Rome III criteria2:
- Presence of 2 or more of the following symptoms:
- Straining during ≥25% of defecations
- Lumpy or hard stools in ≥25% of defecations
- Sensation of incomplete evacuation for ≥25% of defecations
- Sensation of anorectal obstruction/blockage for ≥25% of defecations
- Manual maneuvers to facilitate ≥25% of defecations (such as digital evacuation, support of the pelvic floor)
- Fewer than 3 bowel movements a week
- Loose stools are rarely present without the use of laxatives
- Criteria must have been met for the previous 3 months with symptom onset at least 6 months prior to diagnosis
The Bristol scale describes changes in bowel movement consistency3
The Bristol scale can be used to evaluate bowel movements and helps patients describe them in an objective and minimally embarrassing manner.

References: 1. Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144(1):211-217. doi:10.1053/j.gastro.2012.10.029 2. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gasteroenterol. 2014;109(Suppl 1):S2-S26. 3. Lewis SJ, Heaton KW. Stool form scale as useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32(9):920-924. doi: 10.3109/00365529709011203
Comparison of OTC occasional constipation treatments
OTC laxatives work in different ways

Click for more details about each treatment >
The trademarks depicted herein are owned by their respective owners.
References: 1. Hammer HF, Santa Ana CA, Schiller LR, Fordtran JS. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J Clin Invest. 1989;84(4):1056-1062. doi:10.1172/ JCI114267 2. Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol. 2011;25(Suppl B):16B-21B. 3. DiPalma JA, DeRidder PH, Orlando RC, et al. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95(2):446-450. doi:10.1111/j.1572-0241.2000.01765.x 4. DiPalma JA, Cleveland MB, McGowan J, Herrera JL. A comparison of polyethylene glycol laxative and placebo for relief of constipation from constipating medications. South Med J. 2007;100(11):1085-1090. doi:10.1097/SMJ.0b013e318157ec8f 5. Fiorini K, Sato S, Schlachta CM, Alkhamesi NA. A comparative review of common laxatives in the treatment of constipation. Minerva Chir. 2017;72(3):265-273. doi:10.23736/S0026-4733.17.07236-4
Not all OTC constipation treatments offer both effective results and few side effects
References: 1. MiraLAX® Drug Facts, Bayer Healthcare.2. Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol. 2011;25(Suppl B):16B-21B. 3. Schiller LR, Emmett M, Santa Ana CA, Fordtran JS. Osmotic effects of polyethylene glycol. Gastroenterology. 1988;94(4):933-941. doi:10.1016/0016-5085(88)90550-1 4. Hammer HF, Santa Ana CA, Schiller LR, Fordtran JS. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J Clin Invest. 1989;84(4):1056-1062. doi:10.1172/JCI114267 5. DiPalma JA, DeRidder PH, Orlando RC, et al. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95(2):446-450. doi:10.1111/j.1572-0241.2000.01765.x 6. Phillips® Milk of Magnesia Drug Facts. Bayer Consumer Health. 7. Fiorini K, Sato S, Schlachta CM, Alkhamesi NA. A comparative review of common laxatives in the treatment of constipation. Minerva Chir. 2017;72(3):265-273. doi:10.23736/S0026-4733.17.07236-4 8. Dulcolax® Laxative Tablets Drugs Facts. Chattem, Inc. 9. ex-lax® Regular Strength Drug Facts. GSK. 10. Senokot (sennosides) Drug Summary. Accessed December 5, 2022. https://pdr.net/drug-summary/Senokot-sennosides-3182.84 11. Dulcolax. December 5, 2022. https://www.drugs.com/dulcolax.html 12. Metamucil Psyllium Fiber Supplement. Accessed December 5, 2022. https://www.metamucil.com/en-us/products/fiber-powders/sugar-free- orange-smooth 13. Metamucil FAQs. Accessed December 5, 2022. https://www.metamucil.com/en-us/faqs/metamucil-faqs 14. Colace® Regular Strength Product Information. Accessed December 5, 2022. https:// www.colacecapsules.com/products/colace-regular-strength/ 15. Phillips® Stool Softener Liquid Gels Drug Facts. Bayer Consumer Health.
The trademarks depicted herein are owned by their respective owners.
Medications that may cause constipation
Many commonly used medications have constipation as a side effect. Depending on patients' existing medical concerns, some current treatment regimens may be the cause of their constipation symptoms. Note that patients are advised to talk to their doctor if they experience constipation due to medication.
This represents a small sample of brand name products, some of which may no longer be available. The trademarks depicted in this table are the property of their respective owners.
References: 1. Aluminum hydroxide; magnesium hydroxide; simethicone. Accessed May 7, 2024. https://my.clevelandclinic.org/health/drugs/19931-aluminum-hydroxide-magnesium-hydroxide-simethicone-oral-suspension 2. Gaviscon® Tablets. Accessed May 7, 2024. https://www.gaviscon.com/products/tablets.html 3. Depakote®. Prescribing information. Abbvie Inc.; 2021. 4. Catapres-TTS®. Prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc.; 2011. 5. Rytary. Prescribing information. Amneal Pharmaceuticals LLC; 2019. 6. Haldol®. Package insert. Ortho-McNeil Pharmaceutical, Inc.; 2009. 7. Clozaril®. Prescribing information. Novartis Pharmaceuticals Corporation; 2020. 8. Risperdal® Consta®. Prescribing information. Ortho-McNeil-Janssen Pharmaceuticals, Inc.; 2010. 9. Bentyl®. Prescribing Information. Axcan Pharma US, Inc.; 2011. 10. Ditropan XL®. Prescribing information. Janssen Pharmaceuticals, Inc.; 2016. 11. Detrol® LA. Prescribing information. Pharmacia & Upjohn Company; 2018. 12. Colestid®. Package insert. Pharmacia & Upjohn Company; 2017. 13. Saljoughian M. Pros and cons of calcium supplements. US Pharm. 2015;40(9):HS-28-HS-32. Accessed April 23, 2024. https://www.uspharmacist.com/article/pros-and-cons-of-calcium-supplements 14. Alimta. Prescribing information. Eli Lilly and Company; 2004. 15. Paraplatin®. Package insert. Bristol-Myers Squibb Company; 2010. 16. Furosemide. Package insert. Hospira, Inc.; 2016. 17. Aldactazide®. Package insert. G.D. Searle; 2014. 18. Dyazide. Prescribing information. GlaxoSmithKline; 2020. 19. Wharton S, Davies M, Dicker D, et al. Managing the gastrointestinal side effects of GLP-1receptor agonists in obesity: recommendations for clinical practice. Postgrad Med. 2022;134(1):14-19. doi:10.1080/00325481.2021.2002616 20. Slow Fe® Drug Facts. GSK. 21. MS Contin®. Prescribing information. Purdue Pharma L.P.; 2016. 22. Butalbital, Acetaminophen, Caffeine, and Codeine Phosphate. Prescribing information. Teva Pharmaceuticals USA, Inc.; 2019. 23. Percocet®. Package insert. Par Pharmaceutical; 2020. 24. OxyContin®. Prescribing information. Purdue Pharma L.P.; 2018.











