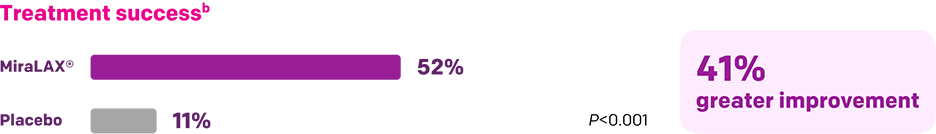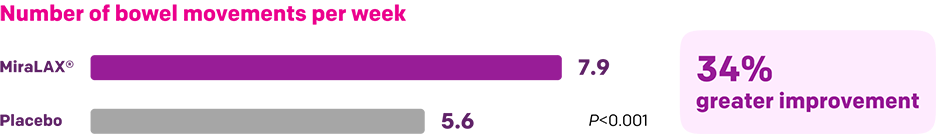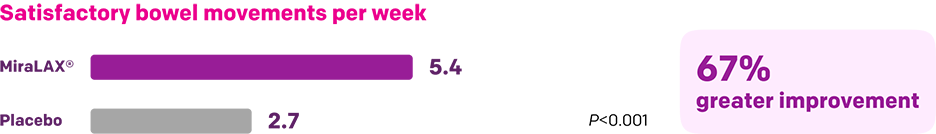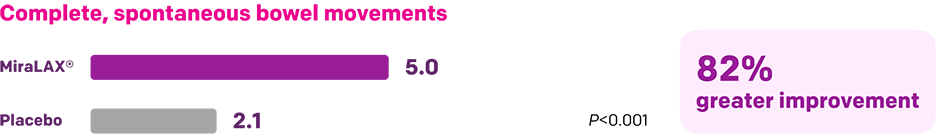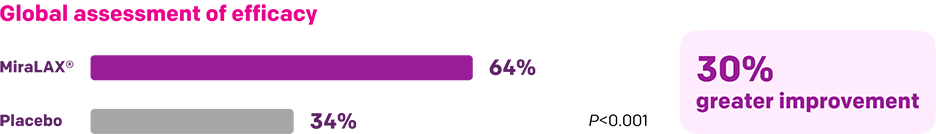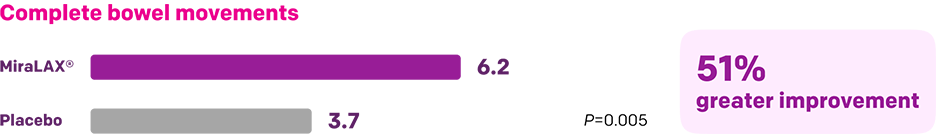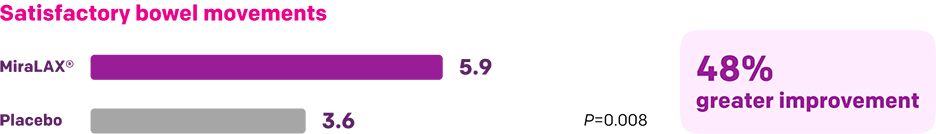Robust Clinical Evidence
Robust Clinical Evidence


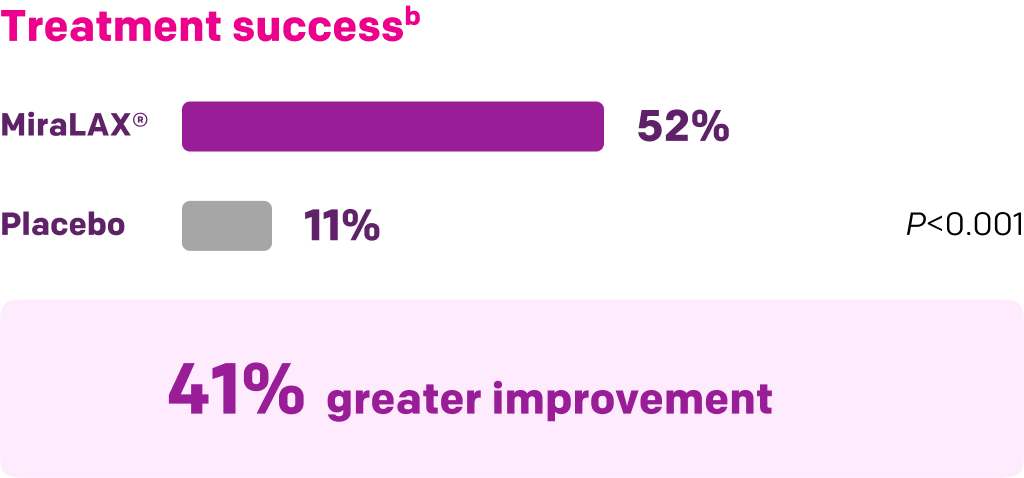
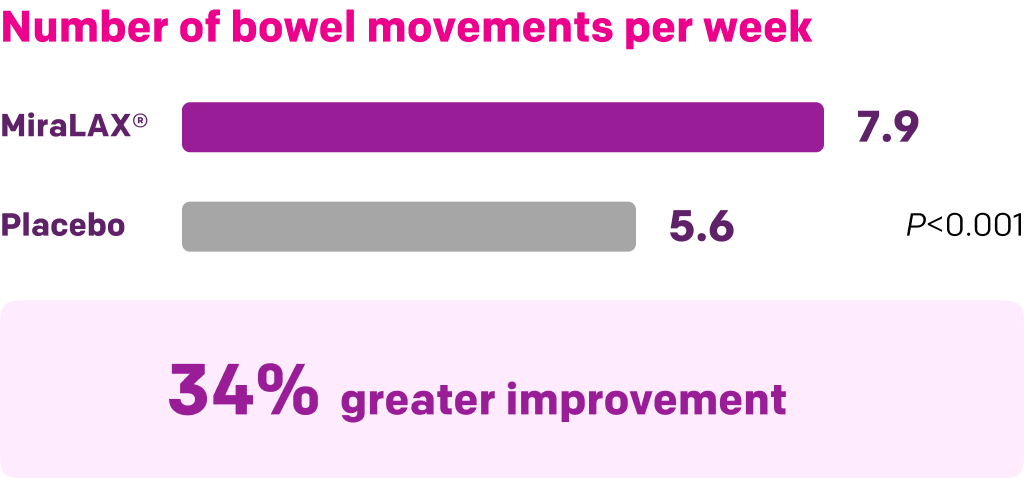
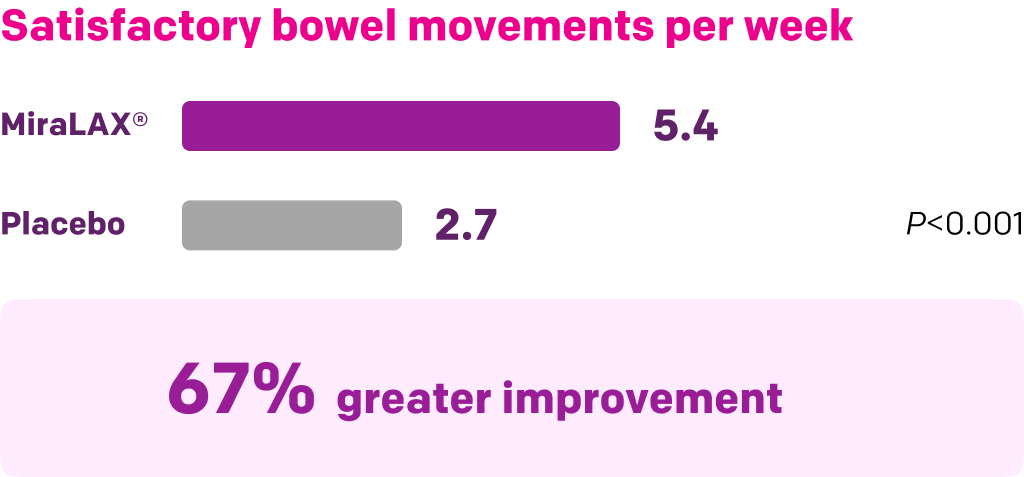
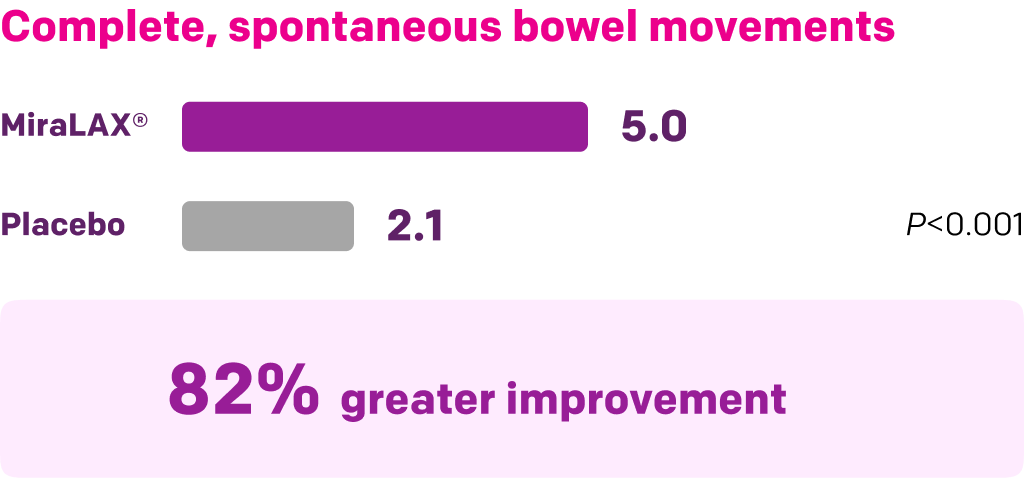
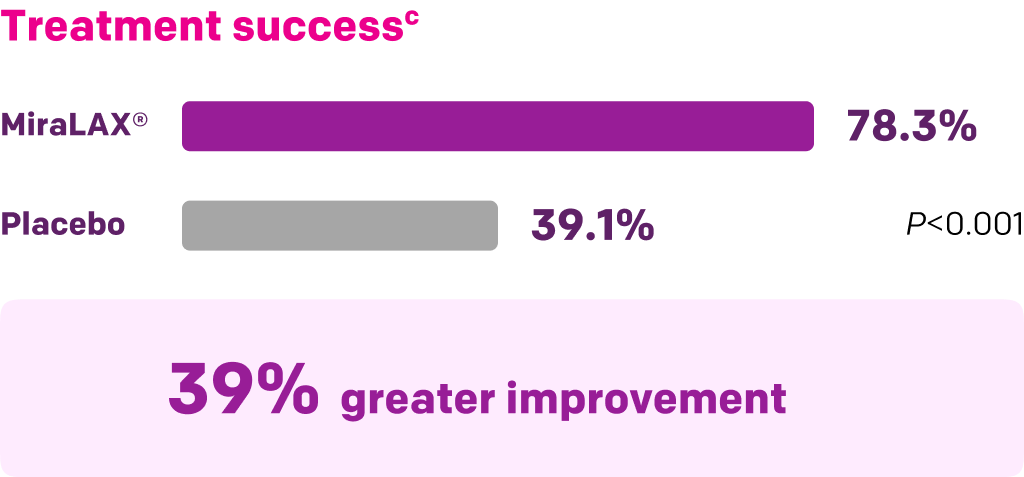
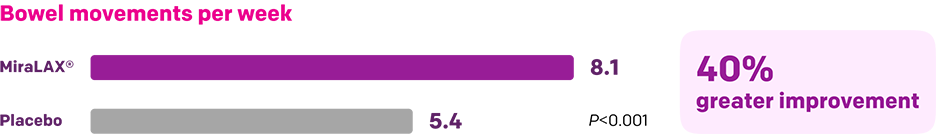
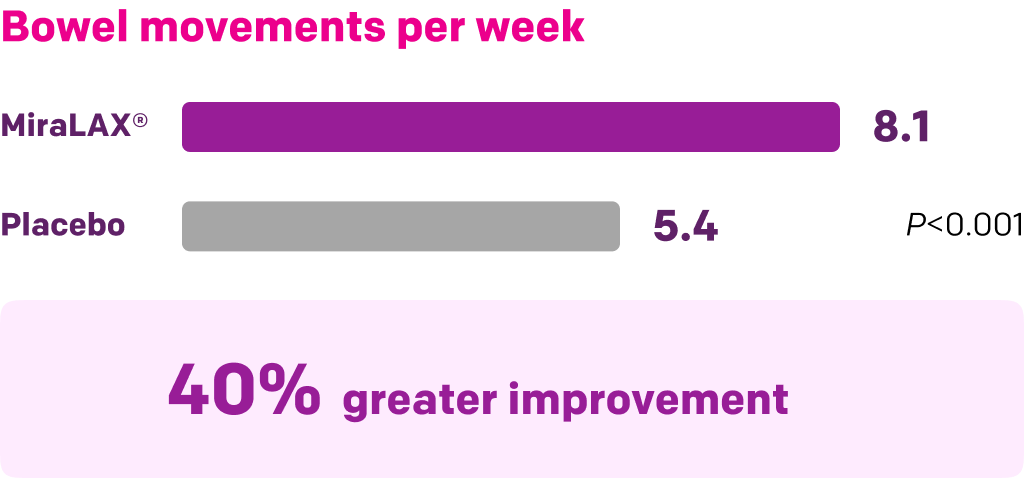
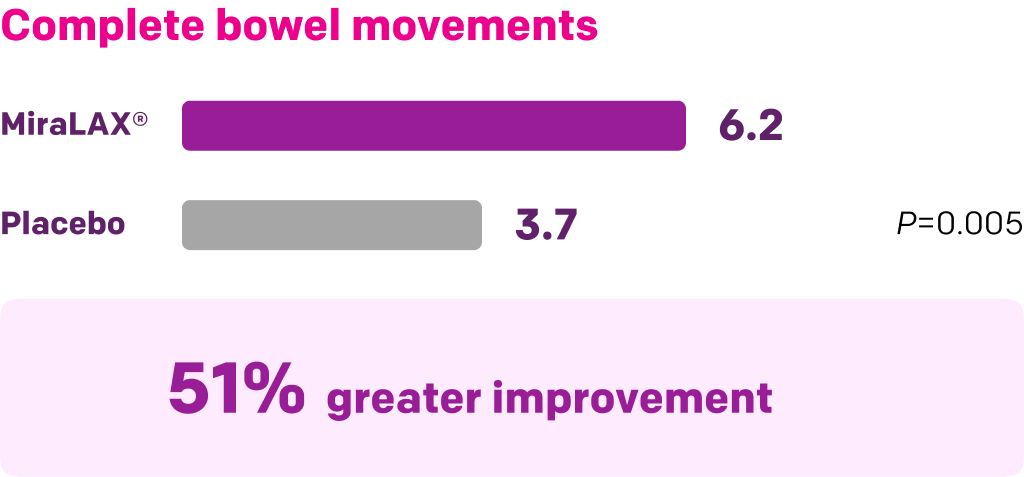
Start with MiraLAX®, proven effective in multiple clinical trials1-5
MiraLAX® Grade A recommendation, Level I evidence, is a higher rating than fibers or stool softeners
Watch Dr. Satish Rao (lead author of the review) present highlights of the findings
PEG = polyethylene glycol.
*A systematic review across PubMed and Embase of randomized controlled trials (≥4-week duration) that evaluated OTC constipation preparations between 2004 and 2020. Studies meeting the following selection criteria were included: 1) randomized controlled trial (placebo or active comparator); 2) parallel or crossover design; 3) established definition of constipation (preferably ROME criteria); 4) minimum duration of 4 weeks of active treatment; 5) well-defined clinical endpoints. Studies were scored using US Preventive Services Task Force criteria (0-5 scale) including randomization, blinding, and withdrawals. The strengths of evidence were adjudicated within each therapeutic category, and recommendations were graded (A, B, C, D, or I) based on level of evidence (Level I, good; Level II, fair; or III, poor).
Summary of key MiraLAX® clinical studies and analyses
Start with MiraLAX®: A first-line OTC laxative recommendation across constipation clinical guidelines
Constipation management guidelines from multiple professional organizations recommend PEG, the active ingredient in MiraLAX®, as a first-line treatment1-3
- Guidelines either don’t mention stool softeners like docusate or don’t recommend them prominently
Recommendations
AAFP=American Academy of Family Physicians; ACG=American College of Gastroenterology; AGA=American Gastroenterological Association; ASCRS=American Society of Colon and Rectal Surgeons; Rome IV 2016=the Rome Foundation’s 2016 publication in Gasteroenterology.
*Consistent, good-quality, patient-oriented evidence.
Click to expand.
References: 1. Paquette IM, Varma M, Ternent C, et al. The American Society of Colon and Rectal Surgeons’ Clinical Practice Guidelines for the Evaluation and Management of Constipation. Dis Colon Rectum. 2016;59(6):479-492. doi:10.1097/DCR.0000000000000599 2. Bharucha AE, Dorn SD, Lembo A, Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013;144(1):211-217. doi:10.1053/j.gastro.2012.10.029 3. Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gasteroenterol. 2014;109(Suppl 1):S2-S26. 4. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150(6):1393-1407. doi:10.1053/j.gastro.2016.02.031 5. Mounsey A, Raleigh M, Wilson A. Management of constipation in older adults. Am Fam Physician. 2015;92(6):500-504.