In 2 recent surveys
Patients and HCPs alike reported a negative quality of life (QoL) impact of constipation1,2
Patient profiles are hypothetical and stories are for illustrative purposes only.
“My golf buddies are fed up waiting for me while I run back to the clubhouse restroom, and I’m fed up with their jokes about my fiber and my flatulence.”
Constipation can cause quality of life issues.2 Start your patients on triple-action MiraLAX® sooner
In a survey of 557 patients in the Knowledge Networks Panel who reported chronic constipation2
73% reported social or personal impairment due to constipation2
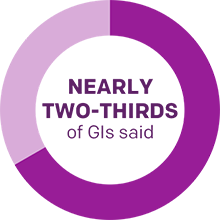
In a recent survey of 300 gastroenterologists1,a
These mechanisms of action are the most important for OTC treatment of OC:
- Colon hydration
- Softening and increasing of stool volume
- Peristalsis that avoids urgency and cramping
and that MiraLAX® provides these more effectively than other OTC treatments
- 3x more GIs chose MiraLAX® than chose fiber supplements as the most effective combination of hydration, stool softening, and gentle peristalsisb
- 1.5x as many GIs said MiraLAX® provides relief free of harsh side effects as said the same about fiber supplementsc
- 92% stated that they believe that MiraLAX® effectively relieves OC without harsh side effects such as bloating, cramping, gas, or urgencyc
- 90% said MiraLAX® is “the OTC laxative that best balances efficacy and tolerability” and is the one “they trust the most”d
aBased on a survey that asked 300 gastroenterologists to rate the important factors in choosing an OTC medication for adults with OC and how well MiraLAX® met those needs. HCPs selected from a set of options, based on their clinical experience. Survey details and data are available upon request.
b75% of GIs rated the degree of negative impact ≥5 on a scale of 1-7.
cGIs rated this benefit of MiraLAX® ≥5 on a scale of 1-7.
dGIs rated the strength of their agreement with these statements ≥5 on a scale of 1-7
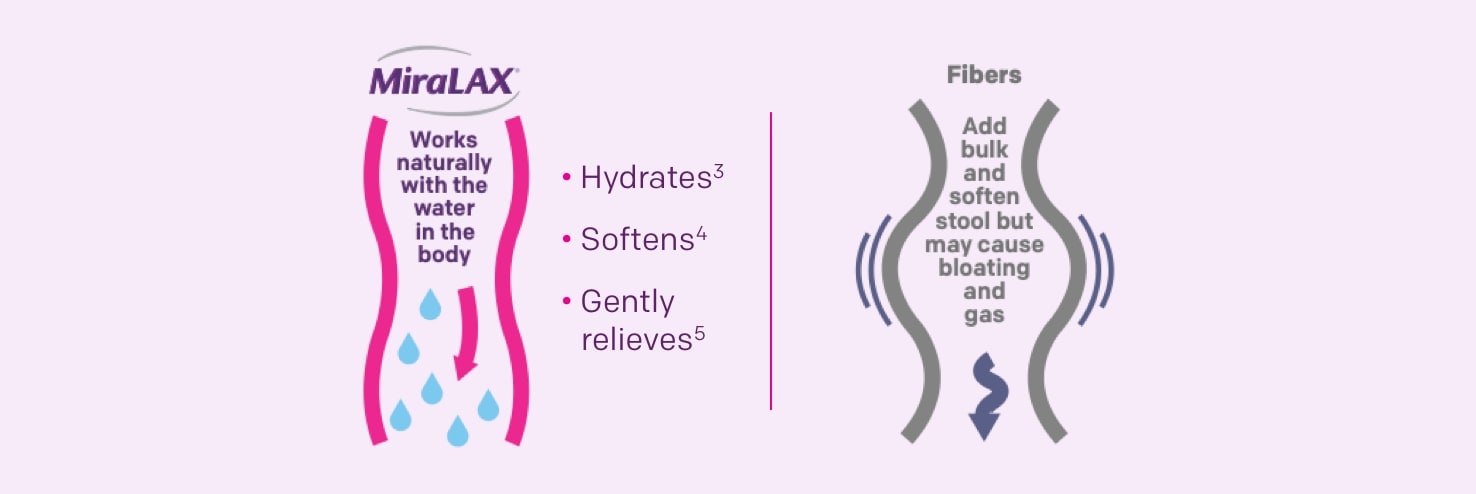
Compare MiraLAX® with fiber
- MiraLAX® binds and retains water in the colon through formation of hydrogen bonds3
- MiraLAX® molecules incorporate water into the stool, softening and increasing volume4
- MiraLAX® promotes gentle peristalsis5 without harsh side effects (colonic nerve stimulation or metabolization by bacteria)4,6,7
- Fiber laxatives metabolize in the intestine, which could cause bloating and gas buildup8
Start your patients like Jack on MiraLAX® sooner:
Its triple action hydrates, softens, and promotes gentle peristalsis3-5
References: 1. Data on file, Bayer Healthcare. 2. Johanson JF, Kralstein J. Chronic constipation: a survey of the patient perspective. Aliment Pharmacol Ther. 2007;25:599–608 3. Schiller LR, Emmett M, Santa Ana CA, Fordtran JS. Osmotic effects of polyethylene glycol. Gastroenterology. 1988;94(4):933-941. doi:10.1016/0016-5085(88)90550-1 4. Hammer HF, Santa Ana CA, Schiller LR, Fordtran JS. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J Clin Invest. 1989;84(4):1056-1062. doi:10.1172/JCI114267 5. Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol. 2011;25(Suppl B):16B-21B. 6. DiPalma JA, DeRidder PH, Orlando RC, et al. A randomized, placebo-controlled, multicenter study of the safety and efficacy of a new polyethylene glycol laxative. Am J Gastroenterol. 2000;95(2):446-450. doi:10.1111/j.1572-0241.2000.01765.x 7. DiPalma JA, Cleveland MB, McGowan J, Herrera JL. A comparison of polyethylene glycol laxative and placebo for relief of constipation from constipating medications. South Med J. 2007;100(11):1085-1090. doi:10.1097/SMJ.0b013e318157ec8f 8. Fiorini K, Sato S, Schlachta CM, Alkhamesi NA. A comparative review of common laxatives in the treatment of constipation. Minerva Chir. 2017;72(3):265-273. doi:10.23736/S0026-4733.17.07236-4